Christopher F. Curtis
formerly of the London School of Hygiene & Tropical Medicine, U.K.

Malaria is by far the most important insect transmitted disease (Gilles and Warrell, 1993). Latest W.H.O. estimates are that there are 300-500 million cases of clinical malaria per year, with 1.4-2.6 million deaths, mainly among African children. Malaria is therefore a major cause of infant mortality and is the only insect borne parasitic disease comparable in impact to the world's major killer transmissable diseases: diarrhea, acute respiratory infections, tuberculosis and AIDS.
There are effective anti-malaria drugs and the W.H.O. emphasizes early diagnosis and prompt treatment of malaria. However, there are major problems of drug resistance, particularly to chloroquine which has been the mainstay of malaria treatment, especially in Africa, because of its low cost and relative freedom from side effects.
There is much interest in the development of malaria vaccines, but the only one which has been extensively field tested only gave a limited degree of protection (Alonso et al., 1994).
Between the 1940s and 1960s malaria eradication was achieved in the USA, USSR, southern Europe and most Caribbean Islands mainly by vector control. Much progress was also made in the Indian subcontinent and parts of South America. Now in the 1990s emphasis needs once again to be placed on vector control.

The vectors of human malaria all belong to the genus Anopheles whose adults (Figure 1) are recognized by their "tail in the air" posture, dappled wings in most tropical species and long pair of palps beside the proboscis in the female.
As in other mosquitoes only the females bite and they use the proteins from a blood meal to produce a batch of eggs. These are laid in relatively clean water, such as in marshes, puddles, irrigation water etc. Unlike other mosquito larvae, those of Anopheles float parallel to the water surface (Figure 2). They develop through 4 larval instars to a short lived, motile pupal stage. The whole process from egg to emergence of the adult from the pupa takes little more than a week at tropical temperatures.
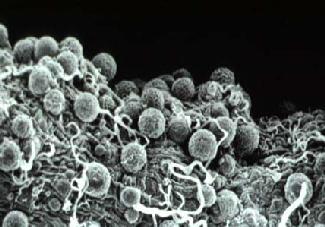
Soon after emergence the adults mate and the female goes in search of its first blood meal. If this contains gametocytes of malaria parasites (which belong to the genus Plasmodium), male and female gametes of the parasite undergo fertilization in the mosquito's stomach, the zygotes develop into oocysts on the outer surface of the stomach wall (Figure 3) and sporozoites develop in the oocysts, over a period of about 12 days, before populating the mosquito's salivary glands. During subsequent feeds the injection of saliva into the host carries sporozoites, which may establish a new malaria infection in the host. During the approximately 12 days required for sporozoite development, a member of most female tropical anopheline mosquito species would be expected, if it survives, to re-visit houses 3 or 4 times to take bloodmeals and thus initiate new cycles of egg development. If the mosquito can be killed at any one of those 3 or 4 house visits it can never develop sporozoites and become a disease vector. It is this fact which is the key to most successful malaria vector control programs which aim to increase the dangers of house visits for adult mosquitoes. Larval control also contributes to some malaria vector control programs, especially where larval sites are limited in extent and are definable - e.g. wells and water tanks in Indian urban areas. In rural areas in the wet tropics Anopheles may breed in every water filled foot- and hoof-print and larval control is an almost hopeless undertaking.

The main method of attacking adult mosquitoes in houses is spraying the inside surfaces of the walls and roof or ceiling with a residual insecticide (Figure 4). This requires teams of trained spray operatives equipped with hand operated spray pumps. The teams must be transported to the villages when they are needed, with adequate supplies of insecticide. The intention of house spraying is that mosquitoes will rest on the insecticide deposit before or after biting and remain long enough to pick up a lethal dose.
The insecticide most widely used for house spraying has been DDT, which has continued to be recommended for this purpose long after it was banned for agricultural use in the USA and many other countries. It has been recommended because of its cheapness per unit weight and its durability, which allows programs to be based on spraying twice a year, or only once in areas with a short annual malaria mosquito season. Arguments about DDT applied to field crops accumulating in food chains are considered inapplicable to DDT sprayed inside houses. However, unfortunately in low income countries it is almost impossible to prevent illicit diversion of insecticides intended for anti-malaria use to farmers. The consequent insecticidal residues in crops at levels unacceptable for the export trade have been an important factor in recent bans of DDT for malaria control in several tropical countries (Curtis, 1994). Some of the claims in the 1960s and 70s about supposed effects of DDT on human health were almost certainly ill-founded. DDT residues in human breast milk have been repeatedly observed, but usually attributed to earlier intake with contaminated food. However, there is recent evidence from South Africa (cited by Curtis, 1994) that, in areas of anti-malaria use of DDT, breast milk contains much higher residues than other areas and in the former areas the intake by a breast-fed baby would greatly exceed the Allowable Daily Intake (which is defined by the WHO and FAO on a lifetime intake basis and so is not readily related to a baby's intake of milk). There is also some evidence (cited by Curtis, 1994) for neurological abnormalities in babies taking in relatively high DDT residues with their milk.
DDT has already been replaced by organophosphate or carbamate insecticides such as malathion or bendiocarb where DDT resistance has been detected, e.g. in Sri Lanka, parts of India, Pakistan, Turkey and Central America. However, these compounds are considerably more expensive to use than DDT, and malathion does not persist well on mud walls.
Pyrethroids such as deltamethrin and lambdacyhalothrin are effective at far lower doses than DDT (c.25 mg/sq. metre compared with 2 gm/sq. metre). Although more expensive per unit weight, these pyrethroids are not much more expensive per house protected per year (Curtis, 1994). They are also much more acceptable to householders because they leave no visible deposit on walls and because they kill nuisance insects such as cockroaches. Therefore rates of refusal of spraying by householders are lower with pyrethroids than with DDT and therefore there is a much better chance of reaching a level of coverage at which the vectorial capacity of the mosquito population will be lowered to a point at which malaria transmission will be interrupted.
Contrary to fears based on experience with other insects such as House Flies, genes for DDT resistance in Anopheles do not generally give cross resistance to pyrethroids. Resistance has been selected to pyrethroids in a few Anopheles populations but so far has not been a major problem in the field. Like DDT, pyrethroids tend to irritate mosquitoes so that they do not rest on deposits for long. However, the pyrethroids paralyze the nervous system so fast that contact for a few minutes is enough to kill, whereas much longer contact is required with DDT and mosquitoes may escape it without picking up a lethal dose. For these reasons, when comparisons have been Control of Malaria Vectors in Africa and Asia made, better malaria control has generally been achieved with pyrethroids than with DDT.
An increasingly popular application of pyrethroids is in the impregnation of bednets (Curtis, 1991). Nets have long been appreciated as a protection against night biting mosquitoes including malaria vectors. However, nets are often torn or hung is such a way that mosquitoes can enter or bite through them. The initial motive for impregnating them with an insecticide, which was safe for close human contact, was to add a chemical barrier to the imperfect physical barrier presented by the net. Studies in experimental huts have proved that pyrethroid impregnation of holed nets makes them function much better in preventing biting of a sleeper than do untreated holed nets. This apparently arises because a treated net kills or irritates and drives away mosquitoes before they have found a hole in the net and entered it.
An additional argument for treating bednets is that they are a rational place in which to deploy a residual insecticide because mosquitoes are attracted to them by the carbon dioxide and body odor emitted by the sleeper. Thus the net acts like a baited trap. In comparison with spraying a family's house, the amount of insecticide needed to treat their nets is much less and synthetic netting is a more favorable substrate for a residual insecticide than is a mud wall. Furthermore bednets intercept mosquitoes in their search for a human host, whereas house spraying pre-supposes that the mosquitoes will rest inside a house before or after feeding, which is not the habit of some Anopheles species, An.dirus Peyton & Harrison. In Hainan Island, China, impregnated bednets were shown to be effective against severe malaria due to Plasmodium falciparum Welch transmitted by An.dirus where DDT spraying was ineffective. Surprisingly, only now is a direct comparison being made between a pyrethroid used for house spraying and for bednet impregnation.
Many of the arguments for impregnation of bednets apply to impregnation of curtains over eaves, windows and doors. The latter method may have the advantages of:-
(i) smaller areas of fabric to treat;
(ii) possibility of using more toxic insecticides which would not be acceptable for intimate contact with people's beds;
(iii) protection of people when they are indoors but before they go to bed.
On the other hand, in rural tropical houses it is difficult to fix curtains (especially eave curtains where roofs are of galvanized iron) so as to make as effective a barrier against mosquito entry as is provided by a bednet. Also bednets can be readily carried by travelers or nomads and can be used over beds or sleeping mats out of doors, as often used by people in very hot countries.
In the world's largest treated bednet program, that in Sichuan Province, China, up to 2.25 million bednets have been treated annually by spraying deltamethrin (Chen et al., 1995). This has been associated with a remarkable decline in the already relatively low level of malaria due to P. vivax (Grassi & Feletti) which is the less serious of the two main species causing human malaria.

In most other projects, bednets are impregnated by dipping in an emulsion of a pyrethroid (Figure 5), drying and re-hanging them. This needs to be done every 6-12 months, or more frequently if the nets are frequently washed. The dipping method is very simple and does not require spray pumps or trained operatives as are required for a house spraying program. Thus, in The Gambia (W. Africa) a National Bednet Impregnation Program has been initiated based on the efforts of village health workers. Monitoring of the first year's results in a sample of the villages showed a 25% reduction in child mortality from all causes (D'Allessandro et al., 1995). Trials are in progress in three other African countries to determine if this result is repeatable. It remains questionable, however, whether this or other forms of vector control will work adequately or sustainably in the heartland of tropical African malaria where transmission rates are much higher than in The Gambia and where large reductions in infective biting rates may still leave the population "saturated" with malaria and where naturally acquired immunity might be expected to fade and counterbalance any benefits of achievable levels of vector control. In such areas integration of vector control with the hoped-for vaccine may be necessary for real progress - the vaccine should aim to replace immunity acquired the hard way via malaria attacks, and the vector control should aim to reduce the level of transmission to a level with which the vaccine can cope.
It is a cause for concern that large scale use of pyrethroid impregnated nets may select for pyrethroid resistance of a physiological kind or may change mosquito behavior so that they bite out of doors before people go indoors to go to bed. There have so far been one or two such reports but the large and sustained projects in China do not appear have produced such problems.
Where Anopheles breeding is sufficiently limited in extent and definable, larval control can make a significant contribution to malaria control. However, it should be recognized that it only works by reducing the density of local vector populations and not by reducing their chances of survival to the dangerous age at which they can carry sporozoites. Furthermore, the effect of localized larval control is easily swamped by immigration from outside the area of control. Thus a high percentage of all productive breeding sites within flight range of the community which it is intended to protect must be found and effectively dealt with. A variety of ways of dealing with them are possible (see Curtis, 1991).
(i) They can be drained or filled so as permanently to remove them as breeding sites. This approach has been applied in industrialized areas in India (Dua et al., 1988).
(ii) Breeding in water tanks can be prevented by screening them - this is legally compulsory in Bombay, India.
(iii) Under some conditions irrigation can be carried out according to a carefully regulated intermittent schedule so that fields are dried once a week and thus larval life cycles cannot be completed.
(iv) The organophosphate insecticide temephos ("Abate") can be applied. This can safely be done even to potable water and there are few places in the world where Anopheles mosquitoes are resistant to it.
(v) The breeding sites may be stocked with larvivorous fish. These are to some extent self-propagating, but sites need to be checked at intervals and those where the fish have died out need to be re-stocked from a fish rearing facility. In some parts of Asia, Grass Carp (Ctenopharyngodon idella) have been used in rice fields which provide a crop of edible fish as well as mosquito control and improvement of rice yields.
(vi) The bacterial toxin from Bacillus thuringiensis israelensis (Bti) can be sprayed into breeding sites as a highly specific agent against mosquito larvae. This is extensively used against larvae of nuisance mosquitoes in Germany and the USA. Unfortunately the toxin is not self-propagating or long lasting in natural breeding sites and frequent re-treatment is unaffordable in most low income countries where the malaria problem exists.
There is considerable interest among biologists, including molecular biologists, in the idea of rendering mosquito populations genetically harmless by introduction of genes which make them non-susceptible to Plasmodium (Collins and Paskewitz, 1994) or divert them from being strongly attracted to biting humans (as is the world's most dangerous malaria vector A. gambiae Giles in Africa) to biting animals. Strains have already been selected which are non-susceptible to Plasmodium and An.gambiae has a sibling species, A. quadriannulatus (Theobald), with which it is inter-crossable in the laboratory and which only bites animals and therefore is not a vector of human malaria.
The real problem with these concepts is not so much producing harmless strains, but propagating their genes extensively given that the capital expenditure to allow mass release from large insect factories would probably only be invested to protect cash crops, such as cattle from the Screw Worm Fly (Cochliomyia hominovorax), whereas protection of the children of the rural poor is not seen as an economically worthwhile activity by those who control economic and political power in the world. Thus if desirable genes are to be spread in Anopheles populations, genetic systems will have to be developed which will reliably cause genes to spread from a small "seeding" of a wild population. Possible candidates for this are transposable elements or the intracellular symbiont Wolbachia.
The idea of creating genes for harmless Anopheles has attracted much attention from laboratory based biologists and this is welcome. However, it is important that this work does not divert resources from the further development and application of methods which already are demonstrably effective at saving lives now, especially the extension of the benefits of impregnated bednets to the hundreds of millions at risk from malaria.
References
- Alonso, P., Smith, T., Armstrong Schellenberg, J.R.M., Masanja, H., Mwankusye, S., Urassa, H., Bastos de Azevedo, I., Chongela, J., Kobero, S., Menendez, C., Hurt, N., Thomas, M.C., Lyimo, E., Weiss, N.A., Hayes, R., Kitua, A.Y., Lopez, M.C., Kilama, W.L., Teuscher, T. & Tanner, M. (1994) Randomized trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 344, 1175-1181.
- Chen Hualiu, Yang Wen, Kang Wuanmin & Liu Chongyi (1995) Large scale spraying of bednets to control mosquito vectors and malaria in Sichuan, China. Bulletin of the World Health Organization 73, 321-328.
- Collins, F.H. & Paskewitz, S.M. (1995) Malaria: current and future prospects for control. Annual Review of Entomology 40, 195-219.
- Curtis, C.F. ed. (1991) Control of Disease Vectors in the Community. Wolfe, London.
- Curtis, C.F. (1994) Should DDT continue to be recommended for malaria vector control? Medical & Veterinary Entomology 8, 107-112.
- Dua, V.K., Sharma, V.P. & Sharma, S.K. (1988) Bio-environmental control of malaria in an industrial complex at Hardwar (U.P.), India. Journal of the American Mosquito Association 4, 426-430.
- D'Alessandro, U., Olaleye, B.O., McGuire, W., Langerock, P., Bennett, S., Aikins, M.K., Thomson, M.C., Cham, B.A. & Greenwood, B.M. (1995) Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet program. Lancet 345, 479-483.
- Gilles, H.M. and Warrell, D.A. (1993) Bruce-Chwatt's Essential Malariology, 3rd edition, Edward Arnold, London